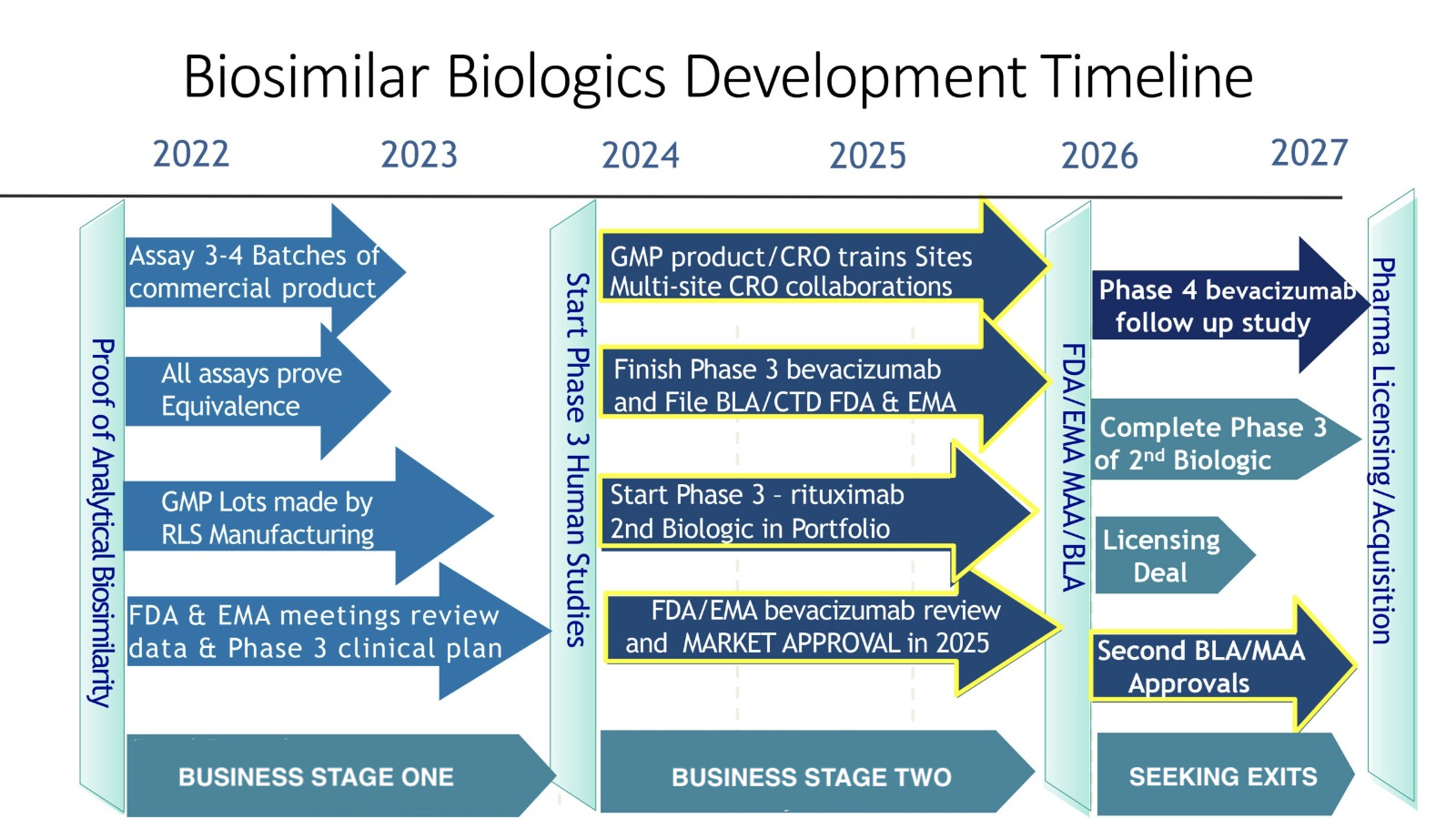
Anew Medical exclusively licensed manufacturing and marketing rights to certain Biologics/Biosimilars in the treatment of cancer.
Biologics
We have entered into (a) an Exclusive License Agreement and (b) an Exclusive License and Manufacturing Agreement in Licensed Territories with Reliance Life Science Private Limited for the licensor’s biosimilars technology for Bevacizumab and Rituximab biosimilar antibodies. The territory for the agreements consists of North America, Europe and Israel.